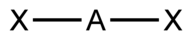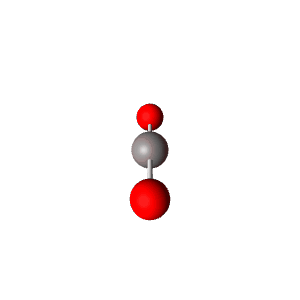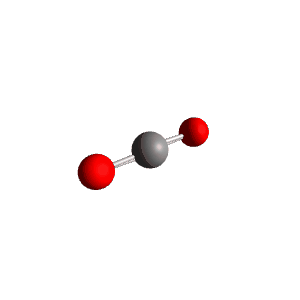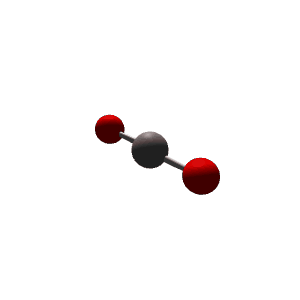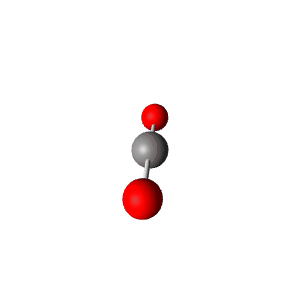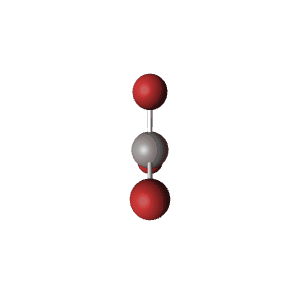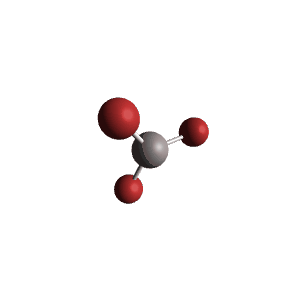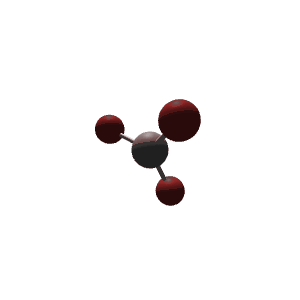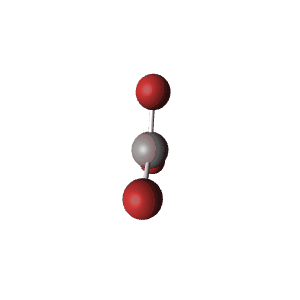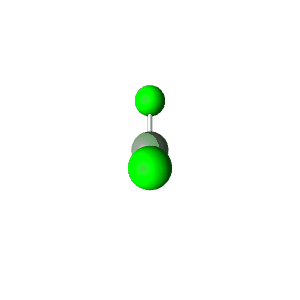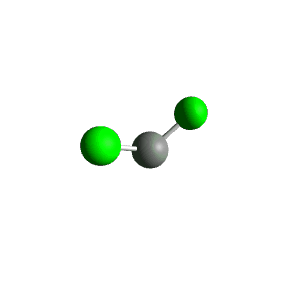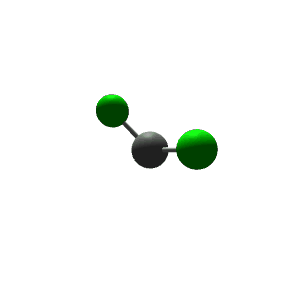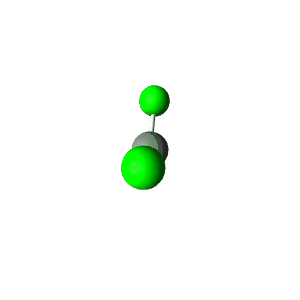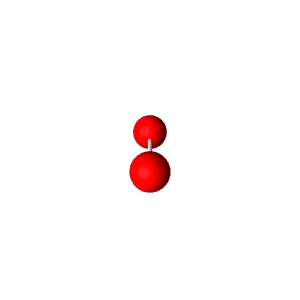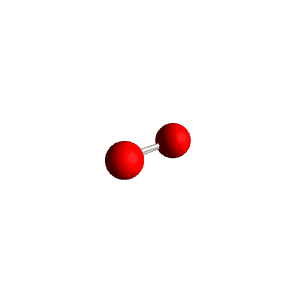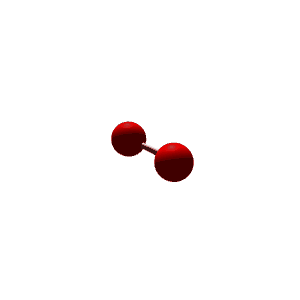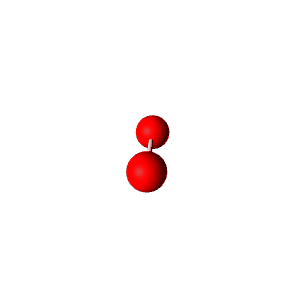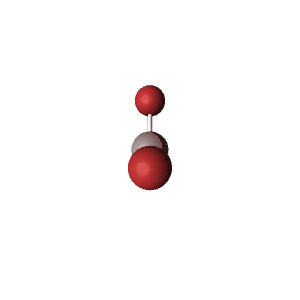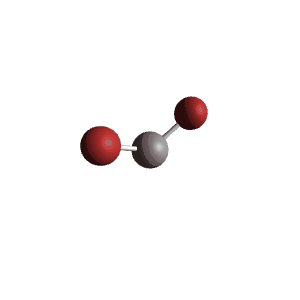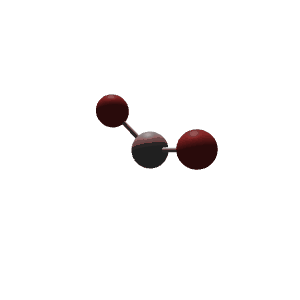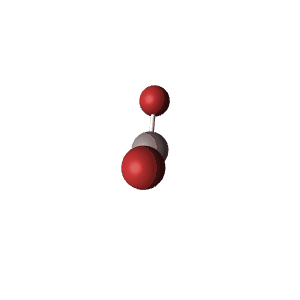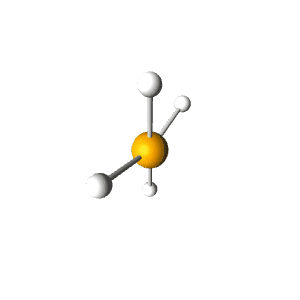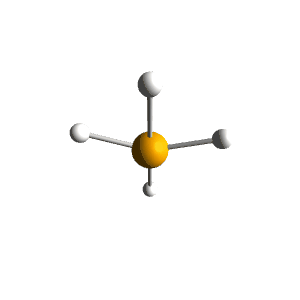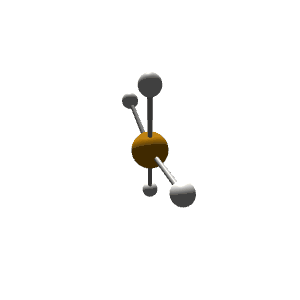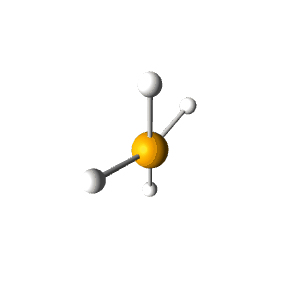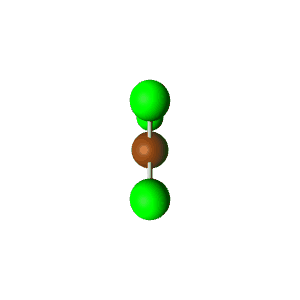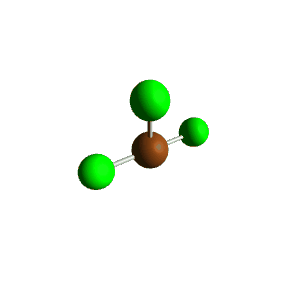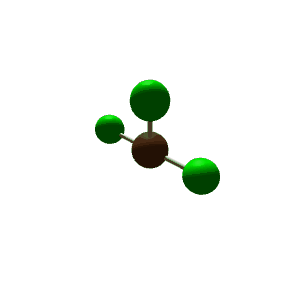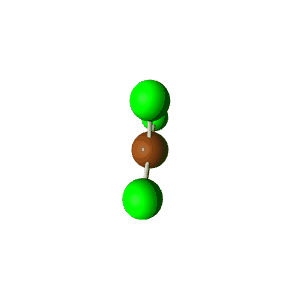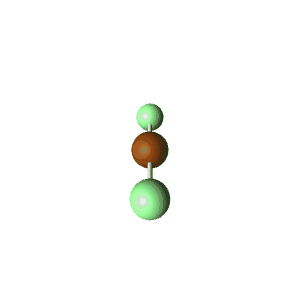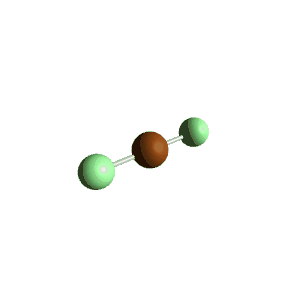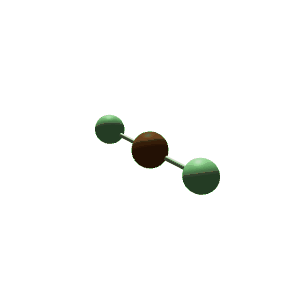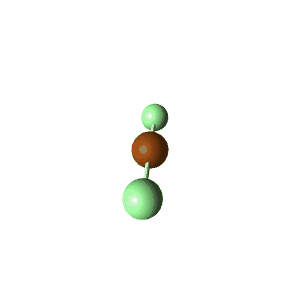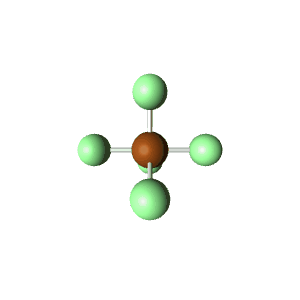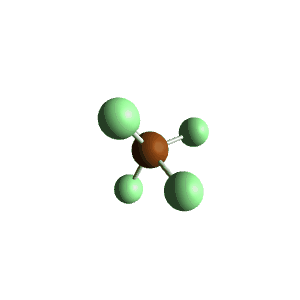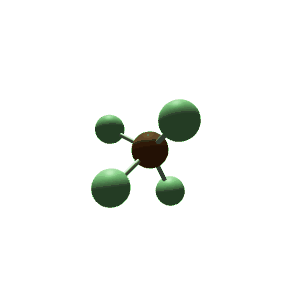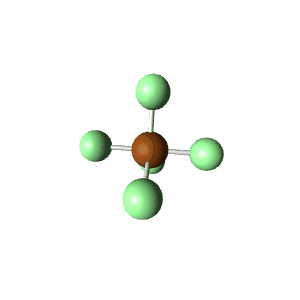| 1 | $\text{AX}$ | $\text{A}\mathrel{\huge{-}}\text{X}$ | 1 | 0 | Linear | Linear | 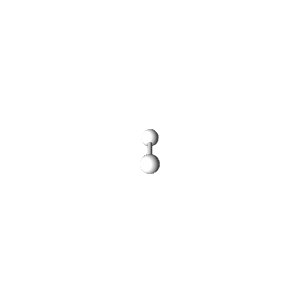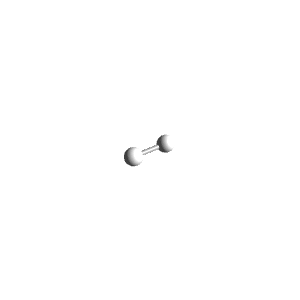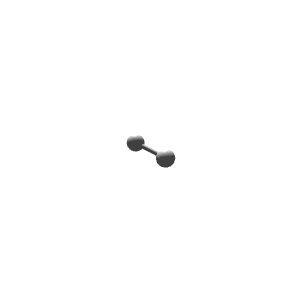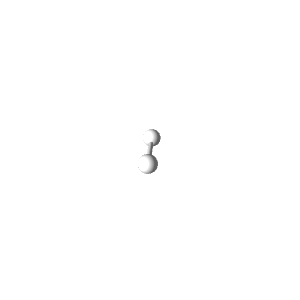 | $s$ | 180° |
| 2 | $\text{AX}_2$ | 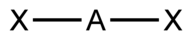 | 2 | 0 | Linear | Linear | 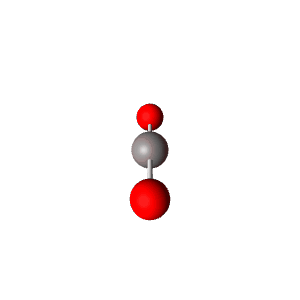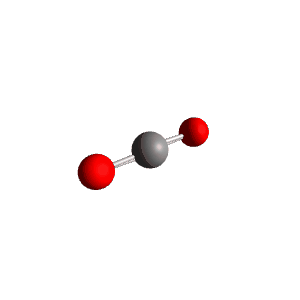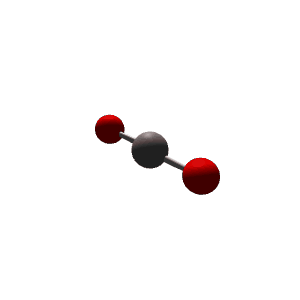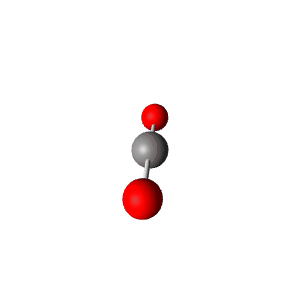 | $sp$ | 180° |
| 2 | $\text{AXE}$ | $\mathrel{\huge{:}}\text{A}\mathrel{\huge{-}}\text{X}$ | 1 | 1 | Linear | Linear | 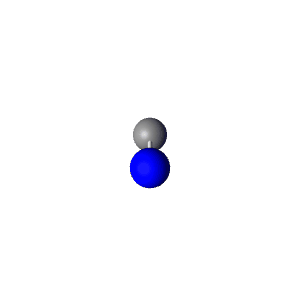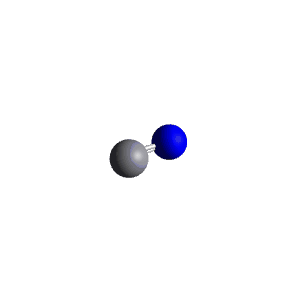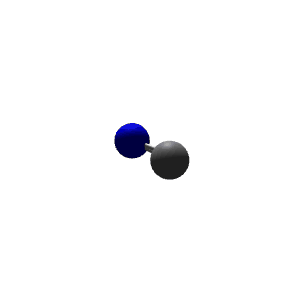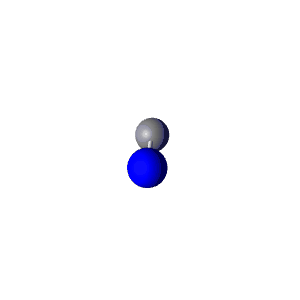 | $sp$ | 180° |
| 3 | $\text{AX}_3$ | 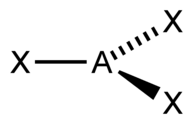 | 3 | 0 | Trigonal Planar | Trigonal Planar | 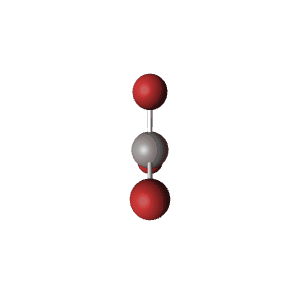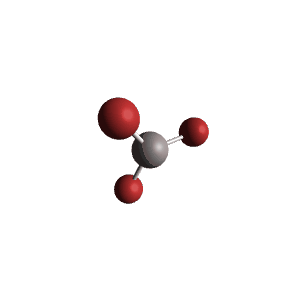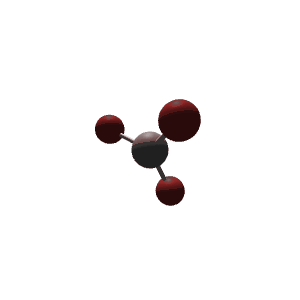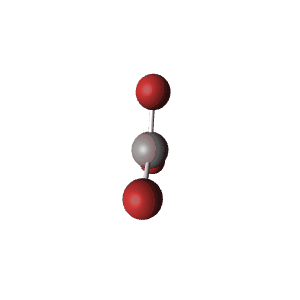 | $sp^2$ | 120° |
| 3 | $\text{AX}_2\text{E}$ | 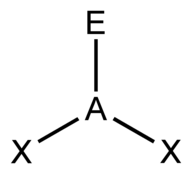 | 2 | 1 | Bent | Trigonal Planar | 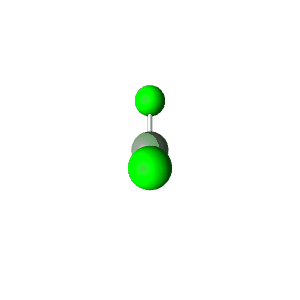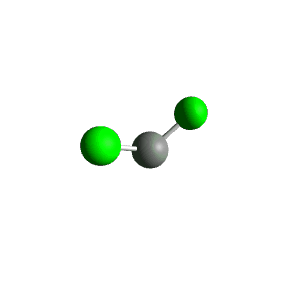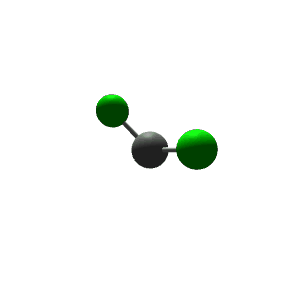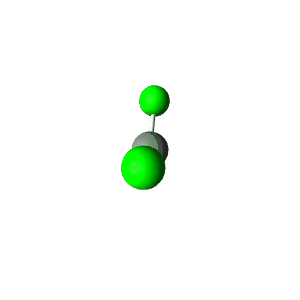 | $sp^2$ | 120° |
| 3 | $\text{AXE}_2$ | $\mathrel{\huge{:}}\overset{\mathrel{\huge\cdot\cdot}}{\text{A}}\mathrel{\huge{-}}\text{X}$ | 1 | 2 | Linear | Trigonal Planar | 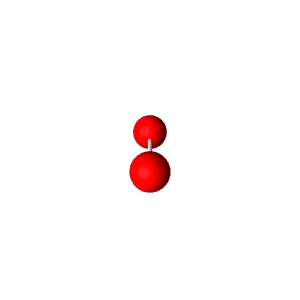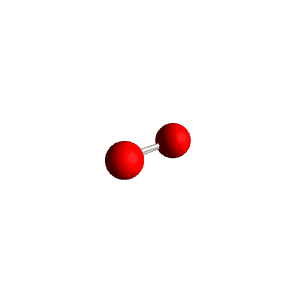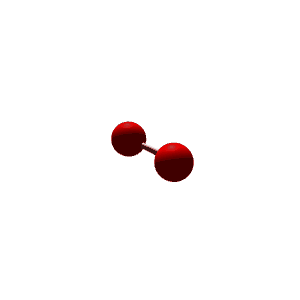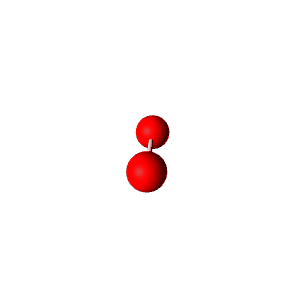 | $sp^2$ | 120° |
| 4 | $\text{AX}_4$ | 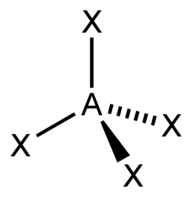 | 4 | 0 | Tetrahedral | Tetrahedral | 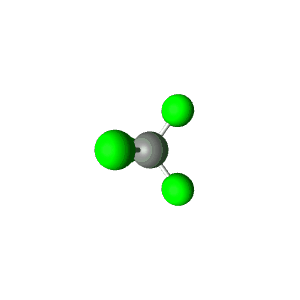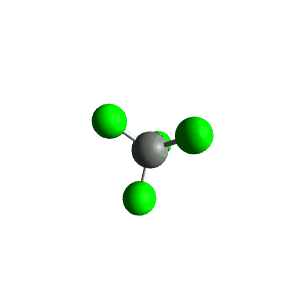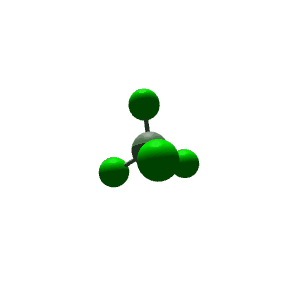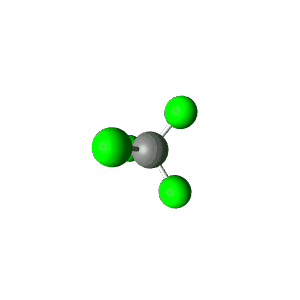 | $sp^3$ | 109.5° |
| 4 | $\text{AX}_3\text{E}$ | 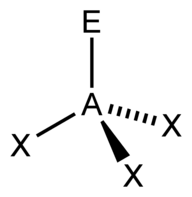 | 3 | 1 | Trigonal Pyramidal | Tetrahedral | 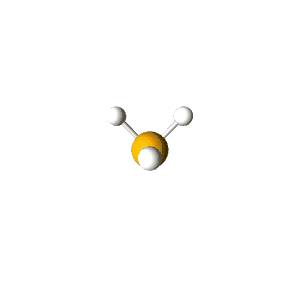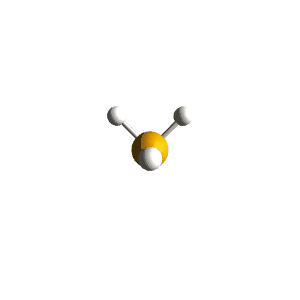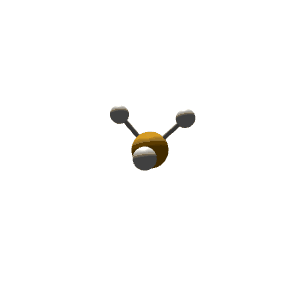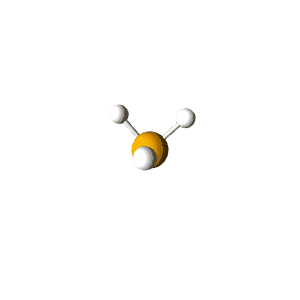 | $sp^3$ | 109.5° |
| 4 | $\text{AX}_2\text{E}_2$ | 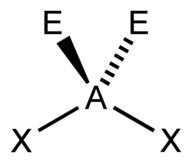 | 2 | 2 | Bent | Tetrahedral | 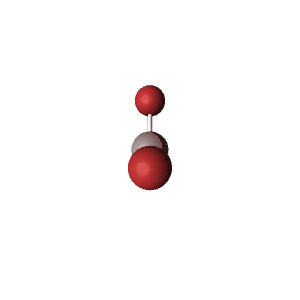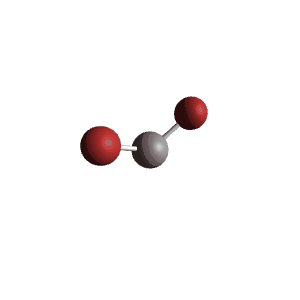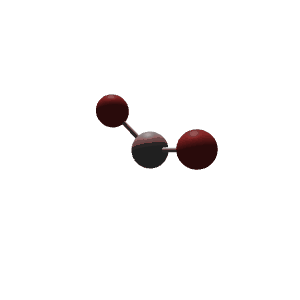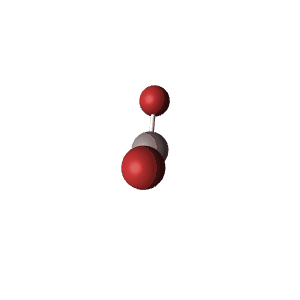 | $sp^3$ | 109.5° |
| 4 | $\text{AXE}_3$ | $\mathrel{\huge{:}} \overset{\mathrel{\huge{\cdot\cdot}}}{\underset{\mathrel{\huge{\cdot\cdot}}}{\text{A}}} \mathrel{\huge{-}} \text{X}$ | 1 | 3 | Linear | Tetrahedral | 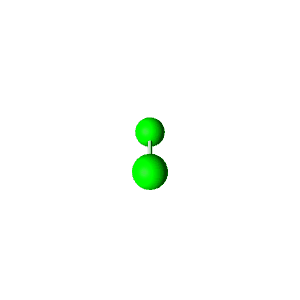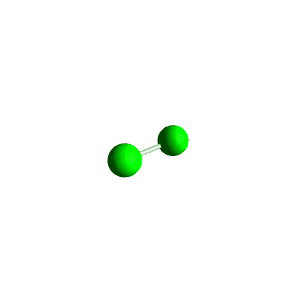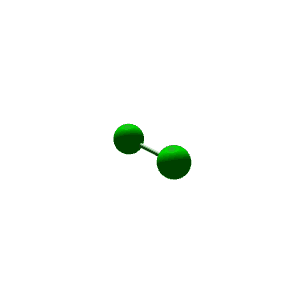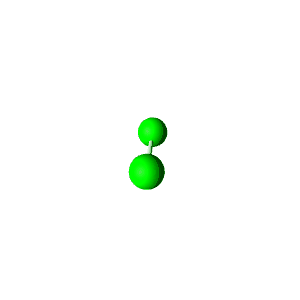 | $sp^3$ | 109.5° |
| 5 | $\text{AX}_5$ | 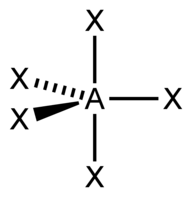 | 5 | 0 | Trigonal Bipyramidal | Trigonal Bipyramidal | 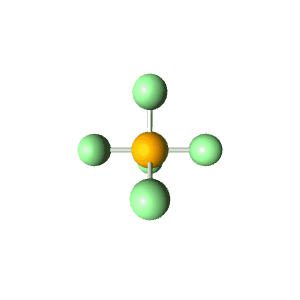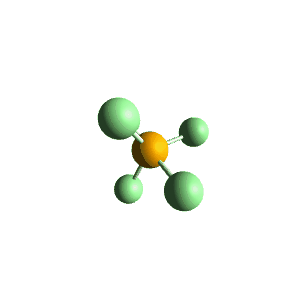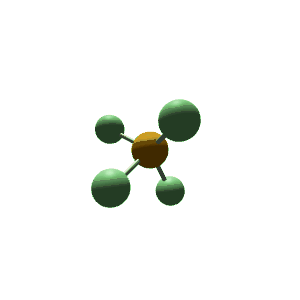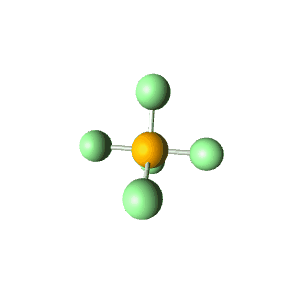 | $sp^3d$ | 90°, 120° |
| 5 | $\text{AX}_4\text{E}$ | 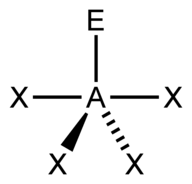 | 4 | 1 | Seesaw | Trigonal Bipyramidal | 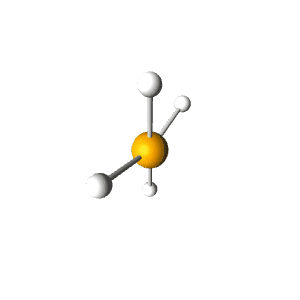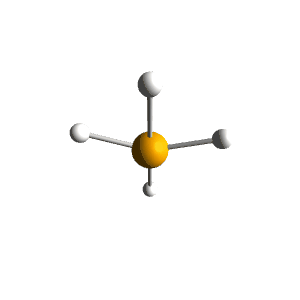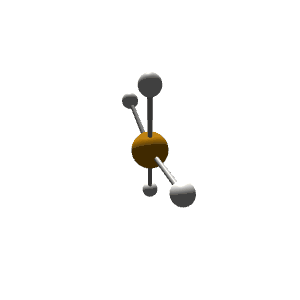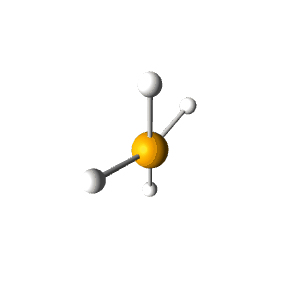 | $sp^3d$ | 90°, 120° |
| 5 | $\text{AX}_3\text{E}_2$ | 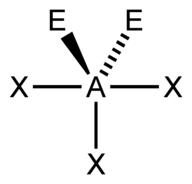 | 3 | 2 | T-Shaped | Trigonal Bipyramidal | 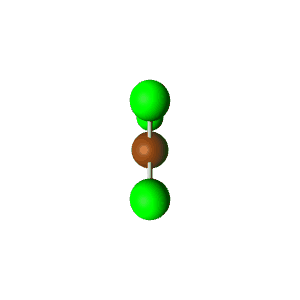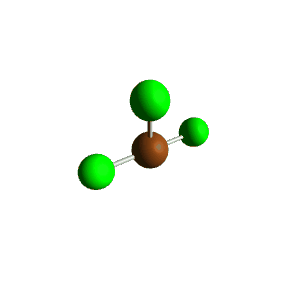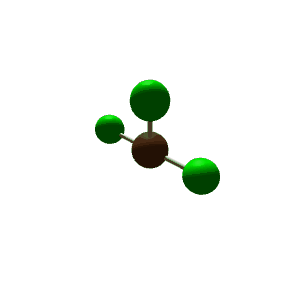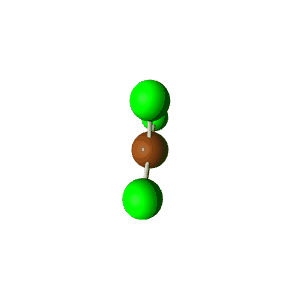 | $sp^3d$ | 90°, 120° |
| 5 | $\text{AX}_2\text{E}_3$ | 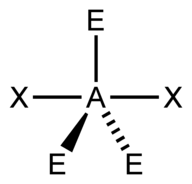 | 2 | 3 | Linear | Trigonal Bipyramidal | 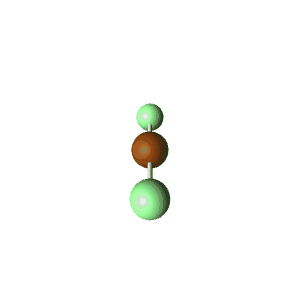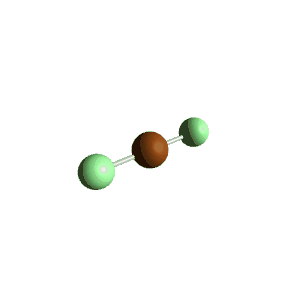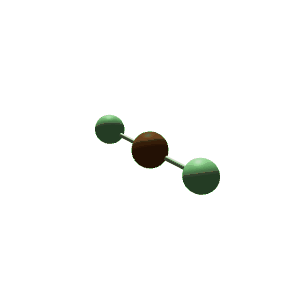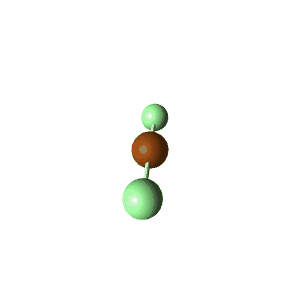 | $sp^3d$ | 90°, 120° |
| 6 | $\text{AX}_6$ | 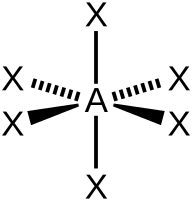 | 6 | 0 | Octahedral | Octahedral | 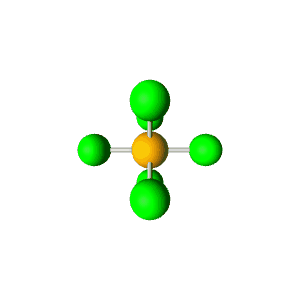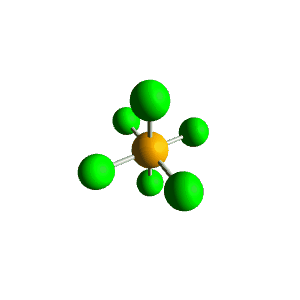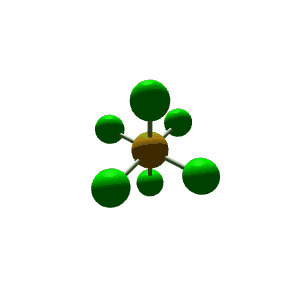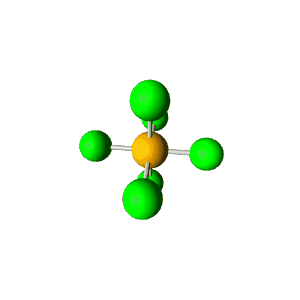 | $sp^3d^2$ | 90° |
| 6 | $\text{AX}_5\text{E}$ | 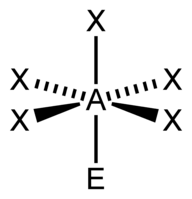 | 5 | 1 | Square Pyramidal | Octahedral | 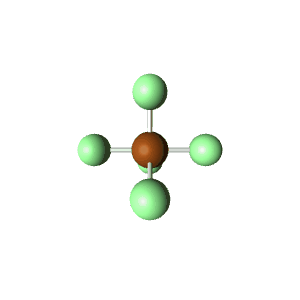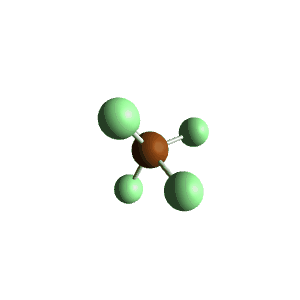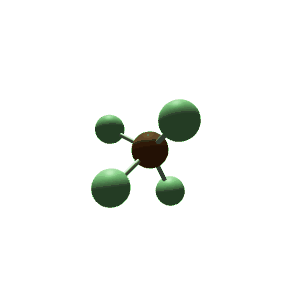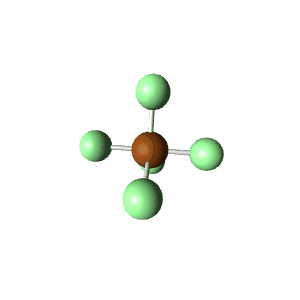 | $sp^3d^2$ | 90° |
| 6 | $\text{AX}_4\text{E}_2$ | 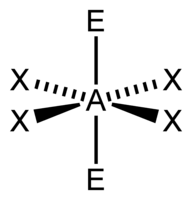 | 4 | 2 | Square Planar | Octahedral | 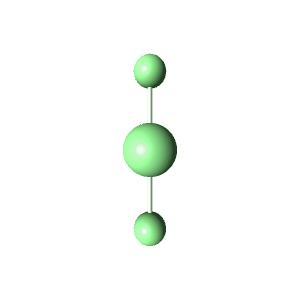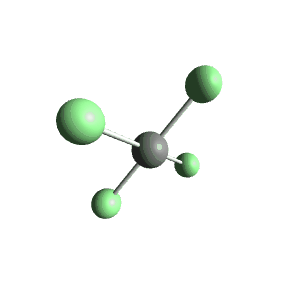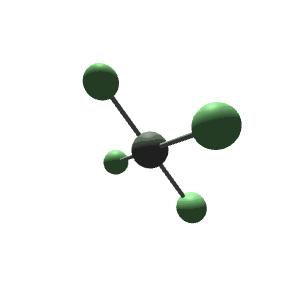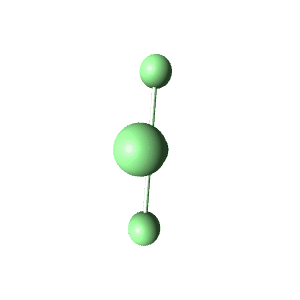 | $sp^3d^2$ | 90° |